Shanghai, China — On 14 November 2023, the one-year follow-up data from the clinical study on MicroPort® MedBot™’s SkyWalker™ in primary total knee arthroplasty (TKA) was published in International Orthopaedics, a journal sponsored by the International Society of Orthopaedic Surgery and Traumatology (SICOT). This marks the first time that SkyWalker™ has conducted a head-to-head, large-sample clinical comparative study with a top international robotic product, drawing conclusions based on long-term follow-up data. The study indicated that the SkyWalker™ robot performed comparably to the leading robot in terms of the accuracy of lower limb alignment, operation time, estimated blood loss, and six month and one year postoperative follow-ups of knee clinical and functional assessments.
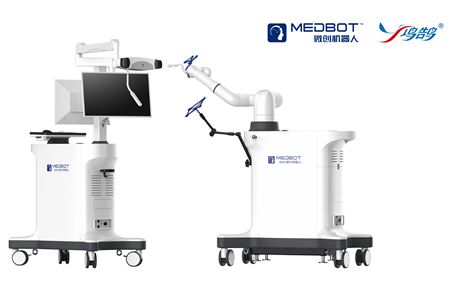
TKA is considered as the gold standard treatment for end-stage osteoarthritis. Accurate implant positioning, appropriate lower limb alignment reconstruction, joint line reconstruction, and gap balance restoration are critical aspects of a successful surgery. This study involved 75 patients, with 30 in the SkyWalker™ group (average age 65.9±5.8 years, 77% female) and 45 in the Control group (average age 67.6±6.8 years, 73% female), showing no significant baseline differences between the two groups.
The study compared the Hip-Knee-Ankle (HKA) from the osteotomy plan to the actual postoperation in both groups, showing no significant statistical difference (177.46±3.04 vs. 177.91±2.24, P=0.458). Additionally, there were no significant differences in the three key indicators of operation time, blood loss, or postoperative hospital stay between the two groups, where operation times were (122.50±20.50 vs. 117.71±21.95, P=0.352), blood loss (347.69±174.71 vs. 306.81±165.30, P=0.335), and postoperative hospital stay (median: 6, interquartile range: 4.5 to 7 vs. median: 6, interquartile range: 5 to 7, P=0.372), respectively.
The study completed the 6-month and 1-year follow-ups postoperatively. There were no significant differences in key metrics including the range of motion (ROM), Knee Society Score (KSS), the Western Ontario McMaster University Osteoarthritis index (WOMAC), and visual analogue scale (VAS). All patients exhibited good healing of the incision, with no cases of exudation of the nail hole, aseptic loosening of the prosthesis, periprosthetic infection, or periprosthetic fracture, except for one patient in the Control group who was readmitted at nine months postoperatively for persistent periprosthetic discomfort and underwent revision surgery.
The clinical study further validates the high surgical precision of the SkyWalker™ robot in assisting TKA, achieving favorable postoperative effectiveness.
While continuously enhancing product performance, the MicroPort® team is dedicated to providing comprehensive professional services, including ongoing reinforcement of clinical education and training, customer support, and clinical assistance. Mr. Yu Liu, Executive Vice President and Chief Business Officer of MicroPort® MedBot™, affirmed, “We will persist in innovation, anchored in evidence-based medicine, delivering precise, efficient, and safe surgical solutions for patients worldwide.”
About SkyWalker™
SkyWalker™ robot is designed for assisting in total knee arthroplasty (TKA) and total hip arthroplasty (THA) surgeries, featuring platform integration, standardization, precision, and personalization. Its preoperative planning system generates personalized prosthetic implantation plans based on patient-specific anatomical characteristics, and the robot collaboratively executes precise bone cutting and grinding during surgery. Combined with MicroPort®'s Evolution® Medial-Pivot Knee System, it emulates the knee's natural stability and movement, providing surgeons with an innovative solution for issues like instability, anterior knee pain, and functional limitations.
Currently, the SkyWalker™ robot has received certifications from China's National Medical Products Administration (NMPA), the U.S. Food and Drug Administration (FDA), the European Conformity (CE), Brazil's National Health Surveillance Agency (ANVISA), and the Australian Therapeutic Goods Administration (TGA).
Reference:
- Hang‑Yu Ping, Hao‑Ming An, Zheng Cao, Shao‑Kui Nan, Hai‑Feng Li, Wei Chai: Efficacy of the newly designed “SkyWalker” robot compared to the MAKO robotic system in primary total knee arthroplasty: a one‑year follow‑up study. International Orthopaedics. https://doi.org/10.1007/s00264-023-06023-1.
-
 2025-11-11SkyWalker® Robotic System Surpasses 500 Robot-Assisted TKA Procedures at Germany’s Krankenhaus Nettetal
2025-11-11SkyWalker® Robotic System Surpasses 500 Robot-Assisted TKA Procedures at Germany’s Krankenhaus Nettetal -
 2025-05-16MicroPort® MedBot™ SkyWalker® Robot Recommended by NICE for Use in the UK NHS
2025-05-16MicroPort® MedBot™ SkyWalker® Robot Recommended by NICE for Use in the UK NHS -
 2024-09-29SkyWalkerTM has received approval for launch by MHLW, achieving comprehensive coverage in key markets!
2024-09-29SkyWalkerTM has received approval for launch by MHLW, achieving comprehensive coverage in key markets!






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.