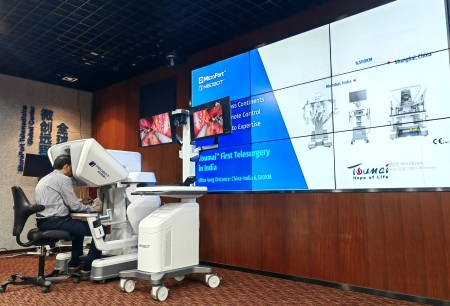
Shanghai, China, 09 October 2025 — MicroPort® MedBot™ announced today that cumulative global commercial orders for its Toumai® Endoscopic Surgical Robot have reached 100 units, with nearly 80 installations completed to date. This positions Toumai® as the leading Chinese-developed endoscopic surgical robot and marks a historic breakthrough in the global commercialization of Chinese surgical robotics.

To date, the total cumulative orders for the core laparoscopic, orthopedic, and endovascular intervention robotic systems under MicroPort® MedBot™ have exceeded 170 units.
Accelerating Global Market Penetration
Toumai® achieved a breakthrough in global both domestic and commercialization within just three years of its launch, becoming the second endoscopic surgical robot globally to scale large-volume deployments. The system has gained access in over 60 countries and regions across Asia, Europe, Africa, North America, South America, and Oceania, with active installations in nearly 40 countries.
MicroPort® MedBot™’s strong global execution, differentiated product performance, and high-touch customer support model have been key to this rapid expansion. The company’s hybrid strategy, combining global academic partnerships, targeted commercialization, and agile regional support, has enabled Toumai® to gain footholds in both advanced and emerging healthcare systems.
Breaking Barriers: Global Adoption in Prestigious Medical Centers
Toumai® continues to break barriers in premium global institutions once dominated by established international brands. Leveraging MicroPort®’s extensive global reach - serving over 20,000 hospitals in 100+ countries - Toumai® has now entered some of the world’s most influential medical centers.
North America: The world’s first FDA-IDE-approved remote robotic surgery was successfully completed using Toumai® in Orlando, Florida.
Europe: Gemelli Hospital in Italy has completed over 50 cases; AZ Delta in Belgium completed 100 cases within 10 weeks and performed Europe’s first remote surgery using Toumai®.
Middle East: Deployed at Cleveland Clinic Abu Dhabi, one of the region’s top-ranked medical centers.
Africa: With four installations in Morocco and over 500 surgeries performed, Toumai® is accelerating robotic adoption across the continent.
South Asia: Kokilaben Dhirubhai Ambani Hospital in India performed 50+ urology procedures and began multi-specialty expansion within its first month.
Southeast Asia: Rajavithi Hospital in Thailand, the country’s top teaching hospital, has been designated as an official national training center for Toumai®.
Latin America: In Brazil, Hospital Nove de Julho completed the region’s first commercial remote human surgery, a milestone for the region’s digital surgery transformation.
Building an International Surgical Training Ecosystem
Toumai® is at the center of a growing international training and education network:
In Europe, leading institutions such as ORSI and IRCAD support academic adoption.
In the Middle East and Africa, Turkey acts as a regional training hub.
In Latin America, Brazil anchors a growing network of partners.
In Asia-Pacific, Thailand’s collaboration with the Ministry of Health is driving a national training model.
This global academic network supports the safe, effective adoption of robotic surgery across diverse geographies and healthcare systems.
From Product to Ecosystem: Defining the Future of Surgical Robotics
The milestone of 100 Toumai® orders represents more than product success; it reflects the emergence of a scalable ecosystem powered by robotic and remote surgery technologies.
In April 2025, Toumai® became the world’s first surgical robot to receive regulatory approval for commercial remote surgery, a landmark moment that underscored China’s leadership in robotic innovation. The system’s capabilities are now recognized and being studied by peers across the US, Europe, and Japan.
Toumai®’s remote surgery platform has received regulatory clearance in several countries and achieved FDA-IDE approval. With this foundation, MicroPort® MedBot™ is building a three-tier global remote surgery ecosystem that removes geographic barriers, supports international academic collaboration, and increases access to high-quality care in under-resourced regions.
A Future of Sustainable Global Growth
“Remote surgery is not a simple extension of in-room surgery; it is a paradigm shift in medical care delivery,” said Dr. Chao He, President of MicroPort® MedBot™. “By dramatically reducing deployment costs and improving scalability, Toumai® enables sustainable, large-scale implementation of robotic and remote surgery worldwide. With each installation, we are not only delivering a product but building a connected, intelligent ecosystem that redefines the future of surgical care.”
As MicroPort® MedBot™ continues expanding its global remote surgery network, Toumai® is evolving from a standalone device into a platform for collaborative, cross-border innovation. This transformation positions MicroPort® not just as a supplier of surgical systems, but as a co-architect of modern surgical infrastructure and an enabler of smart, equitable healthcare ecosystems.
More information is available at: www.medbotsurgical.com/en
-
 2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population
2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population -
 2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations
2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations -
 2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina
2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.