Shanghai, China—Cathbot (Shanghai) Robot Co., Ltd. (“Shanghai Cathbot™”), a joint venture between Shanghai MicroPort MedBot (Group) Co., Ltd. (“MicroPort® MedBot™”) and Robocath S.A.S (France), recently announced the completion of the registration clinical trial for R-ONE™, a vascular interventional surgical robot it introduced to China, making it the first robotic system for vascular intervention to have completed a multicenter clinical trial in the country. During the trial, R-ONE™ was used in a variety of difficult and complex cases such as double-vessel lesions, long diffuse lesions, distorted vascular lesions with large angle kinking and poor coaxiality, calcified lesions, ectopic opening and pseudo-occlusion. The reliability and stability of R-ONE™ was also verified by multiple three consecutive PCI procedures carried out in various medical centers, laying an important foundation for the development of more precise, intelligent and minimally invasive vascular intervention procedures.
The R-ONE™ clinical trial is led by the 301 Hospital in collaboration with Shanxi Provincial Cardiovascular Hospital, Meizhou People's Hospital and People's Hospital of Xinjiang Uygur Autonomous Region. In May 2022, all centers have completed their enrollment trials.
The complexity of lesions places great demands on the operator's precision during vascular interventions. A conventional vascular intervention procedure requires the doctor to manually position the guidewire and catheter using X-ray fluoroscopy. Exposure to x-ray and wearing heavy lead aprons for prolonged periods can cause a variety of occupational diseases.
As a key innovative solution in the field of pan-vascular intervention MicroPort® MedBot™, R-ONE™ features clinical advantages including bionic technology for guidewire translation and rotation, easy exchange and set-up, rapid release and manual switching, sterile barrier coverage, and sub-millimeter stepwise movements, not only making it possible to reproduce operator’s hand movement, but also enhancing the continuity and stability during the delivery of devices such as guidewires/catheters. Thanks to its special minimalist design, it not only reduces the cost of consumables, but also allows for quick setup and release in 3 seconds, making the operation simple and convenient. During the treatment of complex lesions, it can quickly switch catheters/balloons/stents and other instruments with one click to improve the efficiency and shorten the operation time, with the shortest operation time of 11 minutes. The sterile barrier coverage ensures sterile isolation of the guidewire/catheter and other instruments from the robotic arm during operations to ensure patient safety. Through close-up visual comparison and high-precision manipulation of medical instruments, R-ONE™ avoids unnecessary fluoroscopy, reducing radiation exposure and the amount of contrast agent used. The wide-angle full protection screen reduces the radiation exposure to the operator by more than 90%, significantly reducing the surgeon’s risk of occupational radiation exposure. In addition, R-ONE™ is easy to operate with a flat learning curve and can be mastered after minimal training.
Founded in 2009, Robocath develops, manufactures and markets surgical robotic solutions for the treatment of vascular diseases. Shanghai Cathbot™ is a China-based joint venture of MicroPort® MedBot™ and Robocath S.A.S France. Featuring pioneering medical technology, R-ONE™ represents an important advancement for future vascular intervention procedures.
All the medical experts who participated in the clinical trial were impressed by the precision, efficiency, safety and reliability of R-ONE™ demonstrated in clinical application. Professor Yundai Chen, Director of the Department of Cardiology at 301 Hospital, commented, “I am deeply impressed by the accuracy and quick learning curve of R-ONE™. Not only can it complete the positioning of the balloon and stent catheter precisely, it also enables the simultaneous operation of two guidewires, which makes it easier for them to pass through the diseased vessels, hence suitable for complex lesions such as tortuous, calcified, and ectopic openings. This could reduce the risk of intraoperative and postoperative complications, which would benefit both patients and operators.” Professor Jian An, Dean of the Cardiovascular Hospital of Shanxi Province, commented, “The simple and intuitive design of R-ONE™ is very convenient for doctors to operate with. Doctors can complete the intervention by sitting in front of the console and manipulating the devices using only the joysticks. We can get started after short-term training. The learning curve is significantly shortened.” Professor Zhixiong Zhong, Dean of Meizhou People's Hospital, said, “The R-ONE™'s intuitive guidewire cartridge design dramatically reduces the time needed for preoperative preparation, making the procedure more efficient. At the same time, doctors do not need to expose to X-ray while completing the intervention. It significantly reduces intraoperative radiation dose and minimizes doctors’ occupational risk.” Professor Yining Yang, Dean of the People's Hospital of Xinjiang Uygur Autonomous Region, added, “During the clinical trial, R-ONE™ has been very reliable in several consecutive PCI procedures. The R-ONE™ vascular interventional robot features standardized operations, which allow the operator to easily get started and release with precision. Not a single case of complication occurred in the perioperative period. This technology will usher in a new era for cardiovascular intervention characterized by greater intelligence and precision.”
Dr. Philippe Bencteux, Chairman and Founder of Robocath, stated, “The successful completion of the clinical trial is a crucial milestone in the process of bringing R-ONE™ to market. It is the first PCI robotic multi-center trial completed in China. The multi-center clinical trial across different regions has validated the product's ease of use, efficacy and safety. I am very impressed with the commitment of the Cathbot team and the team of researchers. They worked very hard to make it happen on time and to maintain our strategic plans despite the impact of the pandemic. I sincerely thank all of them. Our greatest global ambition is to be able to better treat cardiovascular diseases. R-ONE™ will allow the medical staff to work safely while bringing precision to the intervention. We look forward to the publication of the results of this study soon, which will undoubtedly verify the multiple advantages of the R-ONE™ robotic solution in terms of ease of use, shortened procedure time and reduced radiation exposure. This is the solid first step to the commercialization of R-ONE™ in China.”
Dr. Chao He, President of MicroPort® MedBot™, said, “The completion of the multicenter clinical trial for the R-ONE™ Vascular Interventional Surgical Robot is a major milestone for MicroPort® MedBot™ in the pan-vascular intervention field. It is of great significance in driving the development of more precise and intelligent vascular interventions. In the future, robots for vascular interventions will be equipped with 5G and AI technologies. They will accomplish more breakthroughs when combined with angiography and hemodynamic monitoring and other technologies. Meanwhile, with the advantage of group-based operation, MicroPort® MedBot™ will partner with more academic experts nationwide to promote high-quality medical resources to primary hospitals, and will provide more patients with precise, intelligent and inclusive robotic surgical solutions.”
-
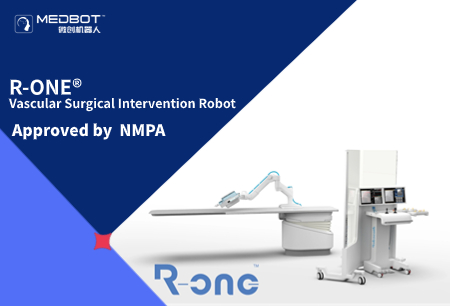 2023-12-13R-ONE® Has Been Approved by The NMPA for Market Launch.
2023-12-13R-ONE® Has Been Approved by The NMPA for Market Launch. -
 2023-07-21Though 2800 km apart, the National First 5G Ultra Remote Robotic Percutaneous Coronary Intervention (PCI) was Completed with the Assistance of R-ONE®
2023-07-21Though 2800 km apart, the National First 5G Ultra Remote Robotic Percutaneous Coronary Intervention (PCI) was Completed with the Assistance of R-ONE® -
 2022-03-03The First Case of Registered Clinical Trial Successfully Completed by R-ONE® Vascular Interventional Surgical Robot in Northwest China
2022-03-03The First Case of Registered Clinical Trial Successfully Completed by R-ONE® Vascular Interventional Surgical Robot in Northwest China






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.