Meizhou, China —— Professor Zhixiong Zhong, Director of Meizhou People's Hospital, and his team successfully completed a case of robot-assisted percutaneous coronary intervention (PCI) in Meizhou People's Hospital on 17 February, 2022 with R-ONE® Vascular Interventional Surgical Robot (R-ONE®) introduced by CathBot (Shanghai) Robot Co., Ltd. ("CathBot®"), a joint venture company established in China jointly by Shanghai MicroPort MedBot (Group) Co., Ltd. and Robocath S.A.S of France. This is the first PCI operation performed with R-ONE® in South China, which further verifies the accuracy and safety of R-ONE®.
The patient suffered gradually deteriorated vague precordial pain and discomfort, went to Meizhou People's Hospital for examination and was diagnosed as having developed severe stenosis in the middle part of left anterior descending branch and needing PCI treatment. Professor Zhixiong Zhong's team decided to adopt R-ONE® to assist the PCI therapy after cautious evaluation. During the procedure, Professor Zhixiong Zhong controlled the guide wire to pass through the diseased blood vessel section through the control handle of R-ONE® smoothly, and then sent the balloon through the control handle to the lesion for vasodilation. After replacement of stent delivery catheter, fine adjustment was conducted on the stent position by sub-millimeter step through the slow feeding mode, the stent was accurately released and lesion was covered, then the procedure was successfully completed. After the procedure, the detection data of on-site radiation measuring instrument showed that the radiation dose of doctors was greatly reduced as compared with those engaged in the traditional PCI treatment.
As physicians need to be exposed to X-ray ionizing radiation in the traditional PCI treatment, they have to wear the heavy lead clothing for a long time to perform the weight-bearing procedure, which is prone to damage to their bone system. R-ONE® is operated by the doctor on the main controller. As it is operated with mechanical assistance, it keeps the doctor farther away from X-ray radiation and reduces the radiation dose on the operator through the lead-prevention screen. Thus the radiation damage to doctors involved can be greatly reduced while the PCI procedure is completed accurately with the remote control of robot.
Professor Zhixiong Zhong commented: "R-ONE® Vascular Interventional Surgical Robot has left a deep impression on me. The balloon and stent can be placed accurately in a robot-assisted procedure, which improves the postoperative effect of patients, reduces the complications, improves the accuracy and quality of procedure, and offers a short learning curve. Meanwhile, R-ONE® significantly reduces the radiation dose on the operators during the procedure, which benefits both the patients and the operators."
Dr. Philippe Bencteux, chairman and founder of Robocath, said: "I am very happy to cooperate with Meizhou People's Hospital and Professor Zhixiong Zhong to in performing the clinical trial. Thanks for their great recognition of the products in clinical application. It has further proved the advantages of R-ONE® in interventional cardiology therapy. We are also full of hope for the development in China, the leading market in the global PCI industry."
Dr. Chao He, president of MicroPort® MedBot®, said: "Thanks to President Zhixiong Zhong of Meizhou People's Hospital and his team for their trust and support to R-ONE®. It is the first PCI operation completed by R-ONE® in South China. Shanghai MicroPort Scientific Corporation Co., Ltd. ranks in the forefront in the field of cardiovascular stent in China, and will cooperate with R-ONE® Vascular Intervention Surgical Robot in the future. Then the doctors will no longer be exposed to radiation, and the patients can share more accurate and efficient integrated solutions."
-
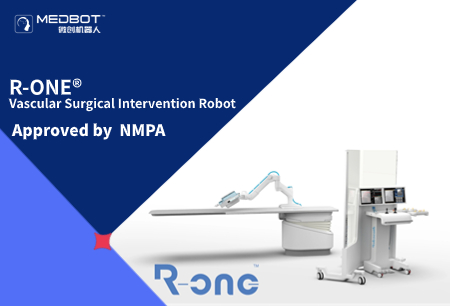 2023-12-13R-ONE® Has Been Approved by The NMPA for Market Launch.
2023-12-13R-ONE® Has Been Approved by The NMPA for Market Launch. -
 2023-07-21Though 2800 km apart, the National First 5G Ultra Remote Robotic Percutaneous Coronary Intervention (PCI) was Completed with the Assistance of R-ONE®
2023-07-21Though 2800 km apart, the National First 5G Ultra Remote Robotic Percutaneous Coronary Intervention (PCI) was Completed with the Assistance of R-ONE® -
 2022-07-17MicroPort® MedBot™ JV’s R-ONE™ Vascular Interventional Surgical Robot Completes Registration Clinical Trial
2022-07-17MicroPort® MedBot™ JV’s R-ONE™ Vascular Interventional Surgical Robot Completes Registration Clinical Trial






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.