Shanghai, China - May 7th, the Toumai® Laparoscopic Surgical Robot system developed by Shanghai MicroPort MedBot (Group) Co., Ltd. (referred to as " MicroPort® MedBot™") has obtained the EU CE certification (MDR), enabling its use in urology, general surgery, thoracic surgery, and gynecological endoscopic surgery. This certification signifies that its stability, clinical effectiveness, safety, and level of technological innovation have been recognized by international authoritative regulatory bodies, laying a solid foundation for further opening up international markets and conducting large-scale clinical applications. It will provide a "Chinese solution" for global surgical robot innovation and usher in a new era.

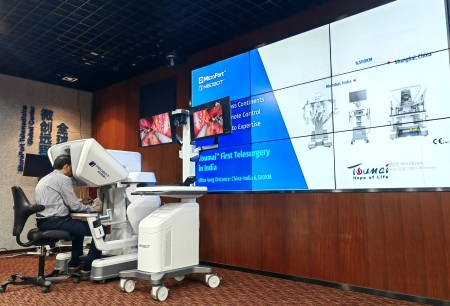
As the first and only domestically produced brand of laparoscopic surgical robot to receive CE certification, the Toumai® robot, with its advanced technological innovation, stable product performance, and comprehensive training service system, has achieved the first overseas sale of a domestically produced laparoscopic surgical robot and smoothly entered clinical applications. This marks a significant breakthrough for domestically produced laparoscopic surgical robots in entering the international market as high-end medical equipment. Currently, the Toumai® robot has completed nearly 3,000 multi-departmental clinical surgeries in over 90 medical institutions at home and abroad, including nearly 200 cases of 5G remote surgery, providing high-quality and affordable medical robot solutions for patients and doctors.
The Toumai® robot features the world's first component of haptic perception in laparoscopic surgical robots - HapticSense®, and has a foot pedal trigger protection and immersion protection. Its advanced imaging system can provide a high-definition field of view with 10x optical zoom, achieving naked-eye 3D imaging. The robotic arm system for performing surgical operations has 7 degrees of freedom, which can stably filter out the physiological tremors of the operator's hands, and the wristed surgical instruments provide unmatched flexibility, precision, and stability, assisting the operator in achieving precise identification, precise dissection, fine excision, and fine suturing reconstruction in narrow cavities. This facilitates the operator to fully demonstrate their surgical expertise and skills, completing refined and highly complex surgeries.
Dr. He Chao, President of MicroPort® MedBot™, stated: "Obtaining the CE certification this time is not only a new breakthrough in our group's globalization strategy but also an important milestone for the domestic laparoscopic surgical robot industry to move towards the global market. We will take this opportunity to further expand our overseas presence, meet clinical needs at home and abroad through high-quality medical robot solutions, contribute 'Chinese intelligence' to the widespread clinical application of surgical robots, and achieve the goal of ' Make Surgery Easier, Safer and Less Invasive'."
-
 2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population
2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population -
 2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations
2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations -
 2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina
2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.