Shanghai, China - On December 1, the Toumai® Remote Surgical System received full-department clinical approval from India’s Central Drugs Standard Control Organisation (CDSCO).
To date, the MicroPort® MedBot’s Toumai® Remote Surgical System has received market approval in nearly 10 countries, including China, India, and Brazil, and was also granted Investigational Device Exemption (IDE) approval by the U.S. FDA.
These milestones extend the system’s coverage to regions representing over 40% of the world’s population.
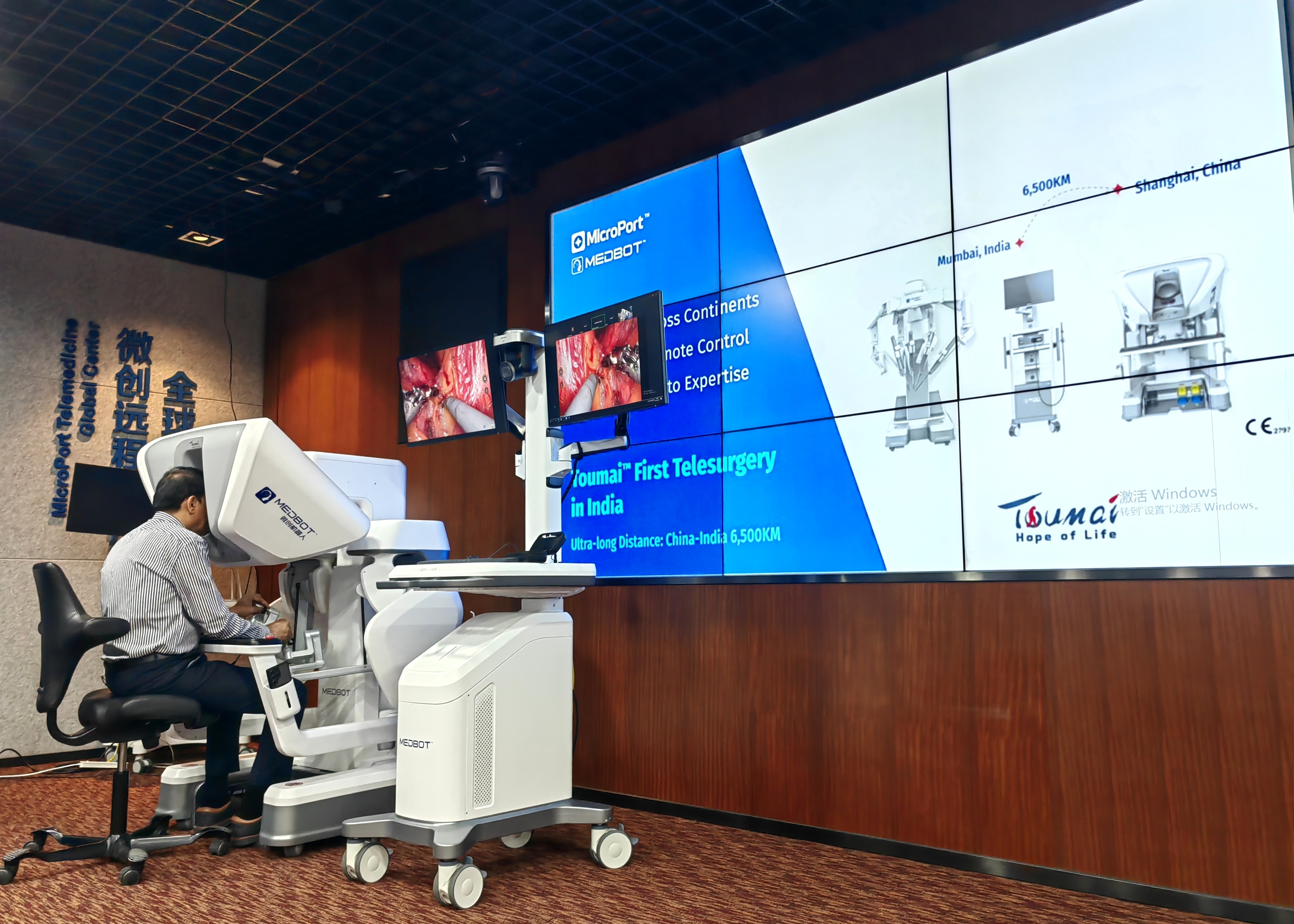
To support large-scale deployment, MicroPort® MedBot has developed an advanced remote surgery technology architecture featuring ultra-low-latency imaging, encrypted data transmission, dynamic network optimization, and multilayer safety assurance.
Toumai® is currently the world’s only commercial remote robotic surgery system with full-department, full-procedure capability. To date, it has supported over 700 procedures across 20+ countries and regions, maintaining a 100% procedural success rate and setting more than 60 world records across performance dimensions.
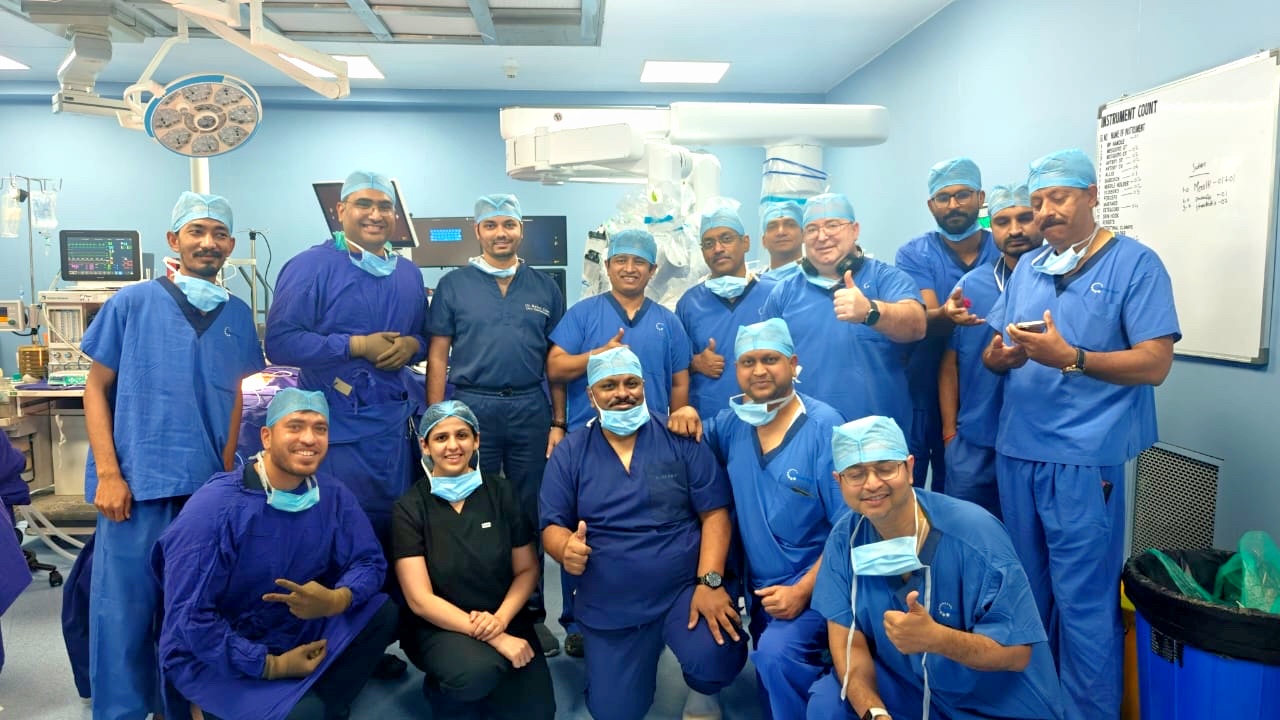
In December, two cross-border urologic surgeries were successfully performed from China to India using Toumai®, with national media highlighting the system’s real-time responsiveness, stability, and precision, comparable to in-person robotic surgery.
-
 2026-02-13Toumai® Laparoscopic Surgical Robot Surpasses 200 Global Commercial Orders
2026-02-13Toumai® Laparoscopic Surgical Robot Surpasses 200 Global Commercial Orders -
 2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations
2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations -
 2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina
2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.