Xiamen, China —— "2022 Oncology Annual Conference of Cancer Research Center of Xiamen University and Series Activities on Centennial Celebration of Biological Sciences of Xiamen University" were held in Xiamen, Fujian Province on April 23, 2022. Shanghai MicroPort MedBot (Group) Co., Ltd. (MicroPort® MedBot®), as one of the leading enterprises of surgery robot, was present at the Conference. A number of renowned Chinese academicians, experts and scholars in the fields of oncology research and therapy gathered at the Conference, and discussed and shared on topics such as clinical pain points, innovative surgical methods and future prospects of oncology.
Representative of MicroPort® MedBot® conducted in-depth discussions with experts attending the annual conference by exchanging on the clinical application and the development prospect of smart surgery in cancer disease therapy, and introduced to them two medical solutions: DFVision® 3D Electronic Laparoscope and Toumai® Laparoscopic Surgical Robot, which are independently developed by MicroPort® MedBot®. The two solutions showed the determination of MicroPort® MedBot® to make ingenuity and strive for excellence in providing Chinese-developed laparoscopic robot-assisted surgery solutions, and to "Make Surgery Easier, Safer and Less Invasive".
The National Medical Products Administration (NMPA) issued the Registration Certificate for Medical Devices to DFVision® in June 2021. The application of independently-developed high-resolution imaging object lens and electronic laparoscopic structure program has not only achieved the full HD presentation of its dual-circuit images, but also reduced the weight of its lens significantly, thus optimizing the operating experience of surgeons. The 3D longitudinal depth vision provided by DFVision® is of great significance to the accurate separation, suture and knot tying in an operation, which has effectively reduced the surgical duration, minimized the operation errors, improved the operation quality and efficiency, truly broken through the limitations in the traditional 2D laparoscopic surgeries. It can be widely used in clinical departments such as general surgery, gynecology and urology.
Toumai®, as a high-end medical device integrated with the multi-disciplinary and high-tech technologies, is composed of a doctor console, a patient surgery platform and an image platform, and NMPA issued to it a Registration Certificate for Medical Devices in January 2022. It adopts the master-slave teleoperation technology so that the surgeons can stay away from the operating table and keep seated during the surgery, thus reducing the burden of doctors. It combines the advantages of robot technology to realize more micro-invasive, accurate, stable and safe operation. It can be widely used in multi-disciplinary surgical procedures, including urological surgeries, and can effectively cooperate with clinicians in implementing the surgical procedures of great difficulty and high precision.
Since the start of the project in 2014, MicroPort® MedBot® has attached great importance to the cooperation with clinics and universities and is committed to researching and producing products closely abreast with the clinical needs. Its product portfolio has already covered five "Golden Solutions": laparoscopic, orthopedic, panvascular, Trans-natural orifice and percutaneous surgical solutions. The Group is committed to meeting the cutting-edge development needs of micro-invasive surgery, applying the cutting-edge research fin dings to industrial integration in the fields of robotics, intelligent control, sensing and information, and innovatively providing a total solution program of smart robot-assisted surgeries that can prolong and reshape life, leading the maturity and development of robotic surgery and shaping the era of ultra-smart surgeries.
-
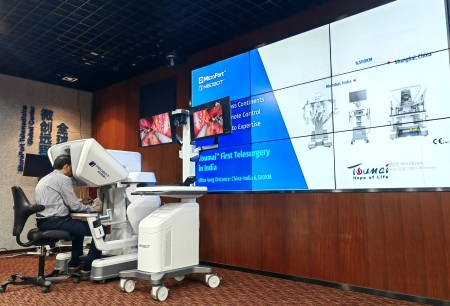 2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population
2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population -
 2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations
2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations -
 2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina
2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.