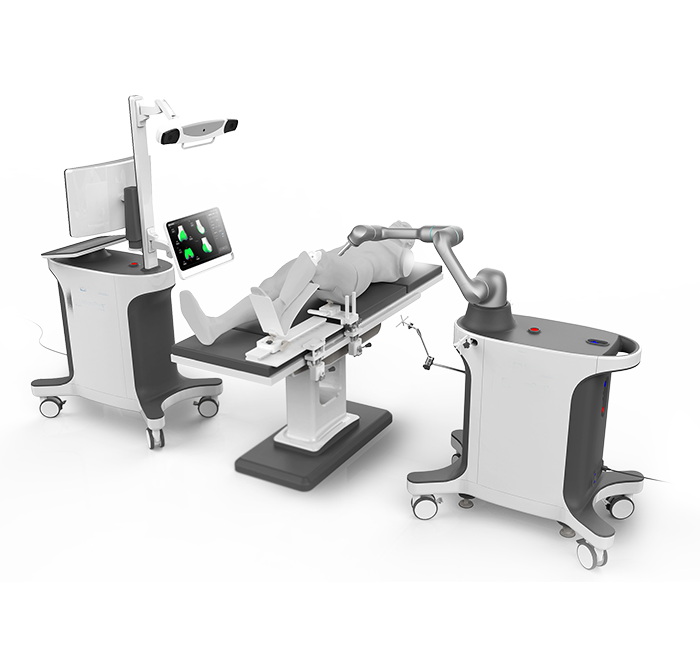
SkyWalker™ was the first and only Chinese surgical robot that has been certified by the National Medical Products Administration of the People’s Republic of China (NMPA) (2022.04), the U.S. Food and Drug Administration (FDA)(2022.07), the Conformite Europeenne(CE)(2022.12), carrying a robot arm independently developed by a Chinese enterprise with its own intellectual property rights.SkyWalker™ has demonstrated the technical advantages in terms of accurate operation, efficient collaboration, safety protection and high compatibility. Its planning system can help doctors make personalized implant plans before surgeries on the basis of preoperative CT scan data and prosthesis model data of patients. During the surgery, it proceeds from the precise positioning of surgical planning, and quickly complete the osteotomy through the registration technology in combination of the independently-developed high dexterity and lightweight robotic arms, thus improving the accuracy and efficiency of operation. SkyWalker™ skips the required location of femoral canal in traditional surgeries and the intramedullary rod implantation during the operation, which can reduce the surgical injury and bleeding, improve the force line after surgery, minimize related complications, and help patients achieve rapid postoperative recovery. SkyWalker™ has won the German iF Design Award, the Italian A'DESIGN Award, the Shanghai Silver Pigeon Award, Shanghai Design 100+,etc..
• Total Knee Arthroplasty (TKA)
• Total Hip Arthroplasty (THA)
NMPA Registration Number 20223010509
FDA Registration Number 510(k) NO: K213873
CE Registration Number MDR 769916 R0000
ANVISA Registration Number 0303010231
ARTG Identifier 413490,412613,412550,410931














 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.