Shanghai, China, 12 March 2025 —— MicroPort® MedBot™ is proud to announce that its Toumai® SP Laparoscopic Surgical Robot has received market approval from the National Medical Products Administration (NMPA) (registration no. 20253010347) in China. This groundbreaking surgical robot is approved for clinical use across a variety of departments, including urology, general surgery, and gynecology.
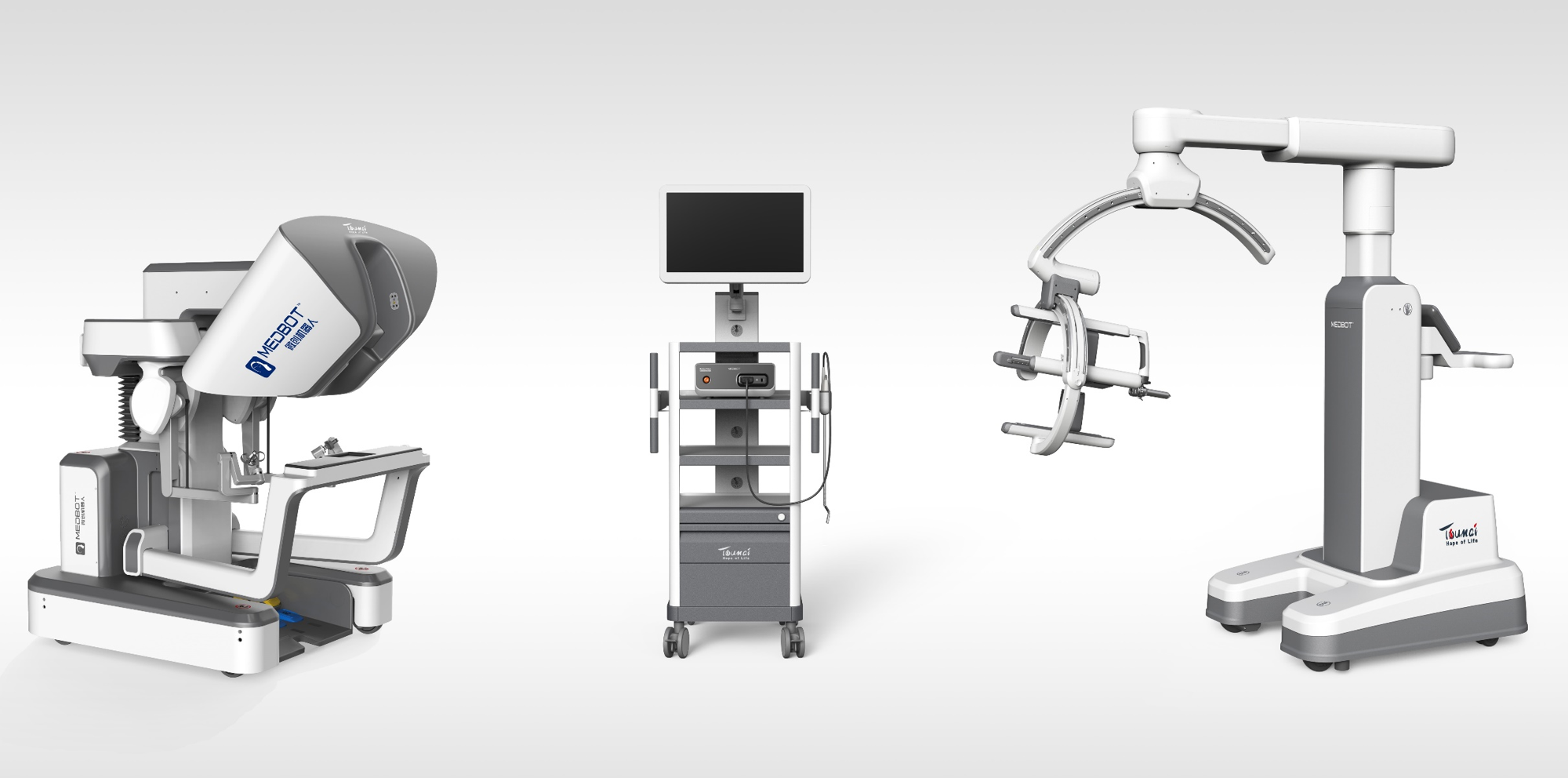
The Toumai® SP boasts an innovative, highly integrated single-port design that enables a 2.5cm incision at the belly button, through which three snake-shaped instruments and a snake-shaped endoscope can be inserted. This compact approach excels in complex environments, such as narrow spaces and intricate anatomical structures.
With its small footprint, high adjustability, and minimal space requirements, the robot is ideal for operating rooms with limited space, improving adaptability in the surgical environment.
Other key features of Toumai® SP include:
Multi-degree-of-freedom Snake-shaped Instruments: Toumai® SP features a 3D high-definition flexible electronic endoscope designed with a multi-degree-of-freedom snake-shaped structure. This design provides a wide field of view and eliminates blind spots. With high-quality imaging, low latency, dual-lens synchronous focusing, and personalized pupil distance calibration, it ensures stable images and smooth operation. The endoscope also offers multiple control modes, an extended lifespan, and helps reduce surgical costs.
Crossed Dual C-arm: Toumai® SP adopts crossed dual C-arm technology, enabling a safer fixed-point design. This allows surgeon to precisely control dual robotic arms through a single minimally invasive incision, supporting full abdominal and pelvic surgeries with enhanced flexibility. By eliminating the need for patient repositioning, it helps reduce the risk of tissue damage.
This approval marks the launch of MicroPort® MedBot™’s fifth surgical robot, further expanding its portfolio of innovative robotic solutions across various specialties and surgical approaches.
-
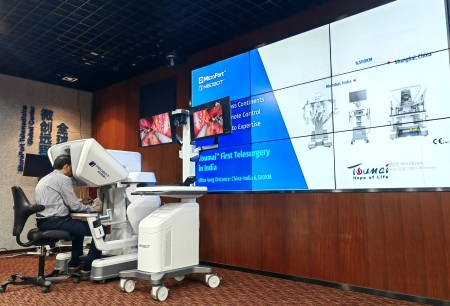 2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population
2026-01-06Toumai® Remote Surgical System Now Approved in Nearly 10 Countries, Reaching Over 40% of the Global Population -
 2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations
2025-12-24Toumai® Becomes the First Chinese Surgical Robot to Achieve the Milestone of 100 Commercial Installations -
 2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina
2025-12-05Toumai®-Assisted Robotic Ovarian Tissue Autotransplantation Successfully Performed for the First Time in Argentina






 Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178
Hu ICP Bei No. 20013662 HGWA Bei No. 31011502015178 " are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.
" are registered trademarks of Shanghai MicroPort Medical (Group) Co., Ltd.” . They have been authorized to be used by Shanghai Microport Medbot (Group) Co., Ltd., and no other party shall use such trademarks without prior written permission thereof.